by Greg Piper
Federal health officials face a growing hurdle in their quest to persuade Americans of all ages and risk profiles to get updated COVID-19 boosters: strong proponents of vaccination.
From New England to the Bay Area, researchers voiced concerns to mainstream science and health publications in recent days that the one-size-fits-all model may be backfiring.
Such experts also questioned whether updated jabs, based on receding Omicron subvariants, will meaningfully benefit Americans outside narrow ranges, if at all.
The Food and Drug Administration (FDA) brushed aside those reservations and didn’t even wait for its own advisory committee to evaluate the performance of new boosters before issuing emergency use authorizations (EUAs) for Pfizer and Moderna products on Monday.
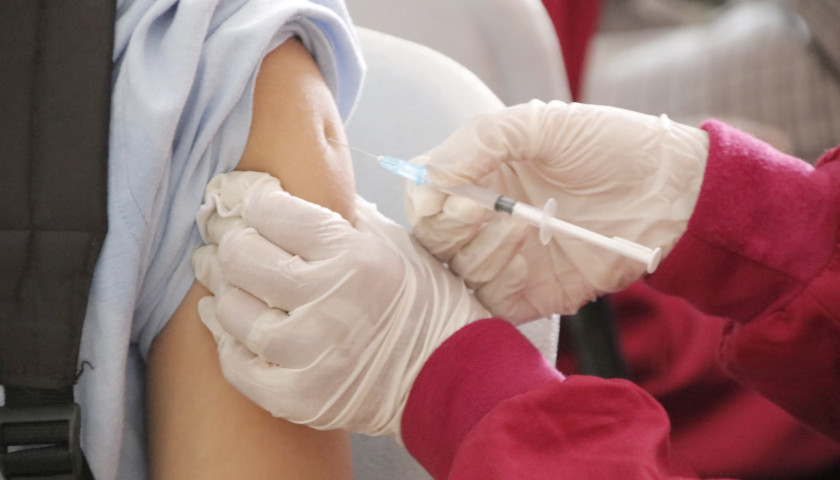
All Americans are eligible for one to three doses, depending on age and vaccination history, despite the FDA’s lackluster description of the new formulations, which are “expected to provide good protection” against “currently circulating variants.”
“Dr. Paul Offit, a pediatrician, vaccine and infectious diseases expert, and vaccine adviser to the Food and Drug Administration, said most healthy young Americans do not require an additional COVID-19 booster shot.” https://t.co/NiwCbWk5ZQ
— Rand Paul (@RandPaul) September 11, 2023
Weill Cornell Medical College immunologist John Moore told Just the News in Monday, before the FDA announcement, that he favored making boosters available for younger people — with their doctors’ approval — primarily for the “mental well being” of those who “have angst about not getting a regular vaccine boost.”
While boosting them provides “possible (and hard to quantify) benefits” to protect their “older, at risk colleagues/relatives,” Moore wrote in an email, it does not pose “significant health risks” to young people at low risk from COVID, “a line the anti-vaxxers take and thrive on.”
Last month the Department of Health and Human Services awarded more than $1.4 billion to develop new COVID vaccines and therapeutics despite CDC data showing just 1 in 6 Americans, and fewer than half of those over 65, are “up to date” with COVID vaccinations.
That shows the Biden administration wasted “the vast majority” of the two-strain bivalent doses it purchased for $4.9 billion without waiting for human data, Johns Hopkins medical professor and National Academy of Medicine member Marty Makary wrote in The Wall Street Journal.
Unlike flu shots, which are approved each year without randomized trials, most COVID vaccines are built on a novel platform and have “high complication rates” in some groups, Makary said, citing mainstream research.
Former Fox News host Megyn Kelly may be one of those complication victims. Last week the 52-year-old said she developed an “autoimmune issue” after receiving a booster and that her doctor had observed the same correlation in other patients. Kelly now regrets even her original Johnson & Johnson vaccine, for which she expressed “zero qualms” about in April 2021.
Sen. Ron Johnson (R-Wis.) blasted the FDA for withholding safety data, based on its “empirical Bayesian data mining” of the Vaccine Adverse Events Reporting System, by invoking ongoing Freedom of Information Act litigation.
“I have routinely received documents and records that were subject to simultaneous FOIA litigation from federal agencies,” including HHS, Johnson wrote in a Sept. 5 letter. He said the CDC waited nine months after his request to both agencies to tell Johnson it didn’t do that data mining.
The increased scrutiny comes ahead of the CDC’s scheduled Advisory Committee for Immunization Practices vote Tuesday on recommendations for the new Pfizer, Moderna and Novavax formulations. The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) is scheduled to meet next month.
Makary questioned Moderna’s Sept. 7 study of its new formulation because the company didn’t report “efficacy or any follow-up” at its first opportunity, the three-month window.
New study: Moderna's novel XBB.1.5 booster was given to 50 people. No data reported on efficacy or any follow-up. They could have reported 3-month follow-up (trial started~4 mo. ago). Important before the FDA/CDC recommend it to 300M Americans next week.https://t.co/HGoVgLciQd
— Marty Makary MD, MPH (@MartyMakary) September 10, 2023
Over the weekend, critics noted the FDA greenlit Moderna’s next version of Spikevax — its non-EUA vaccine — for marketing while labeling it an “unapproved drug,” possibly as a defensive maneuver against litigation, two weeks ago. The agency defines unapproved as posing “significant risks to patients.”
“This is typical obfuscation that we have seen for the Covid vaccines,” Yale University epidemiologist Harvey Risch told Just the News. He noted the Moderna box for ages 11 and younger is labeled “emergency use authorization” while the 12-and-up box for Spikevax does not carry any label, even though the FDA deems it unapproved.
The Association for the Advancement of Science journal Science published an article Friday on scientists who worry about the feds’ one-size-fits-all messaging.
VRBPAC member Paul Offit, the 72-year-old director of the Vaccine Education Center at Children’s Hospital of Philadelphia and himself a vaccine inventor, said he’s planning to skip a second consecutive booster because of his good health and hybrid immunity.
The CDC has not shown the data for a “broad” recommendation, particularly for adolescents who are otherwise healthy but face the “real side effect” of mRNA vaccine-related heart inflammation, Offit said. He previously disclosed encouraging his 20-something son to avoid boosting.
“You can’t ask people to get a vaccine if you’re trying to prevent serious illness and there’s no clear evidence that you are at risk of serious illness,” Offit said. “When you equate 20-year-olds with 65-year-olds that gives 65-year-olds a different idea of what’s necessary,” Brown University epidemiologist Jennifer Nuzzo said.
“The fall 2023 booster is essentially a widespread medical intervention made with zero data,” as evidenced by the U.K.’s drastically narrowed booster recommendations, University of California San Francisco epidemiologist Vinay Prasad wrote this month.
“I would not advise anyone healthy <65 to get the vaccine” and would actively “discourage all COVID19 vaccination of anyone healthy <30,” especially minors because of their higher risk of myocarditis, the middle-aged doctor said.
Prasad will also personally forgo future COVID jabs until he sees evidence it “lowers the risk of severe disease” in his age group and vaccination status — three doses — as well as getting COVID, he said.
The KFFHN article Monday highlighted the stakes of the CDC’s exact recommendation. While some scientists want a narrow recommendation just for those at high risk, that could mean insurers won’t pay for the shots for other groups.
Weill Cornell’s Moore, who expressed early skepticism that bivalent boosters were an improvement, told KFFHN the new formulations are “not remotely a game changer.” He criticized “editorial FOMO” — fear of missing out — for media hyping the threat of new variants such as the “nothingburger” variant BA.2.86.
Moore was a participant in Novavax trials, telling MedPage Today a year ago he only took Pfizer’s primary-series mRNA vaccine a year earlier “to get vaccine-passport-related documentation for travel.”
He told Just the News he plans to stick with Novavax when he gets boosted next month “ahead of a planned foreign trip,” about a year after his last Novavax dose. “I’m 66, in good health, with no known risk factors,” he said.
Moore recommended that others time their booster around the “holiday/travel period” because it’s likely “their greatest virus exposure” and “any additional protection against infection will last only a few months.”
2/“In the study, 8 mice were given BA.5 booster dose”
“‘To rely only on mouse data (for authorization) would be unprecedented in my knowledge & would certainly raise eyebrows,’ said Dr John Moore, a vaccine/virology expert at Weill Cornell Medicine in NY”https://t.co/9EODsi19rn pic.twitter.com/vwxeamjQQY
— Kulvinder Kaur MD (@dockaurG) August 31, 2022
An FDA spokesperson said she couldn’t answer queries by deadline, pointing Just the News to its press release on EUAs for Pfizer and Moderna. It says the agency considered “input from the FDA’s expert advisors,” meaning which formulation to choose, not the performance of that formulation.
The FDA previously declined to comment to The Epoch Times about Johnson’s letter.
– – –
Greg Piper has covered law and policy for nearly two decades, with a focus on tech companies, civil liberties and higher education.