A Tennessee Department of Health (TDOH) official did not say Friday whether his agency currently offers the Food and Drug Administration’s (FDA’s) fully approved COVID-19 vaccine, which the manufacturer, Pfizer, calls Comirnaty.
TDOH officials did, however, comment on the matter in October.
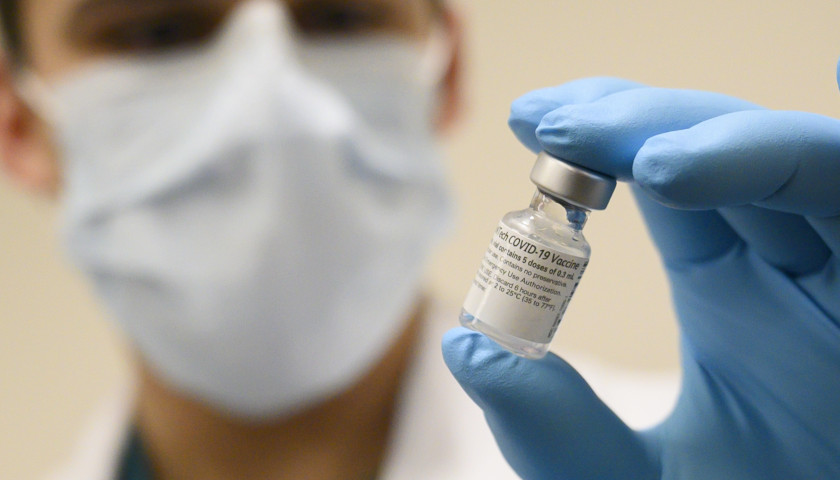
The Tennessee Star on Friday asked the TDOH whether it is shipping Pfizer’s FDA fully approved Comirnaty COVID-19 vaccine or Pfizer’s EUA vaccine, which is not FDA fully approved.
TDOH spokesman Bill Christian responded via email Friday and said he was looking into the matter, but he did not respond again before Friday’s stated deadline.
TDOH officials tweeted in October that the FDA had approved a Pfizer COVID-19 vaccine.
Twitter user @RockyTopSaint that month replied to the TDOH with the following tweet:
“Is the FDA approved Comirnaty available in Tennessee?”
And TDOH officials responded.
“The federal gov has a large amount of the originally-labeled vaccine to be distributed before the branded product is released. The vaccine has not changed. It is the same formulation as the EUA vaccine, but for marketing purposes under the BLA is now referred to as Comirnaty,” TDOH officials tweeted.
The FDA first approved Comirnaty in August, according to that agency’s website.
“The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine, and will now be marketed as Comirnaty, for the prevention of COVID-19 in individuals 16 years of age and older,” according to the FDA’s website.
“Pfizer-BioNTech COVID-19 Vaccine is authorized for emergency use and is available under the EUA as a two-dose primary series for individuals 5 years of age and older, as a third primary series dose for individuals 12 years of age and older who have been determined to have certain kinds of immunocompromise, and as a single booster dose for individuals 16 years of age and older at least six months after completing a primary series of the vaccine.”
Whether Pfizer distributes Comirnaty is unknown, but the company told The Ohio Star this week that it is still shipping the EUA version of the vaccine.
“The Pfizer-BioNTech COVID-19 Vaccine (EUA labeled product) is currently being shipped; however, please be advised that the COMIRNATY and the Pfizer-BioNTech COVID-19 Vaccine have the same formulation and can be used interchangeably,” company officials said via email.
“They are made using the same processes, and there are no differences between them in safety or effectiveness.”
Pfizer did not respond when asked multiple times if Comirnaty is in use anywhere.
Though Pfizer says the vaccines are interchangeable, the FDA concedes that the products are “legally distinct.”
– – –
Chris Butler is an investigative journalist at The Tennessee Star. Follow Chris on Facebook. Email tips to [email protected].
Photo “Pfizer Vaccine” by U.S. Secretary of Defense. CC BY 2.0.